Gallery
 PM Modi visit USA
PM Modi visit USA Only the mirror in my washroom and phone gallery see the crazy me : Sara Khan
Only the mirror in my washroom and phone gallery see the crazy me : Sara Khan Karnataka rain fury: Photos of flooded streets, uprooted trees
Karnataka rain fury: Photos of flooded streets, uprooted trees Cannes 2022: Deepika Padukone stuns at the French Riviera in Sabyasachi outfit
Cannes 2022: Deepika Padukone stuns at the French Riviera in Sabyasachi outfit Ranbir Kapoor And Alia Bhatt's Wedding Pics - Sealed With A Kiss
Ranbir Kapoor And Alia Bhatt's Wedding Pics - Sealed With A Kiss Oscars 2022: Every Academy Award Winner
Oscars 2022: Every Academy Award Winner Shane Warne (1969-2022): Australian cricket legend's life in pictures
Shane Warne (1969-2022): Australian cricket legend's life in pictures Photos: What Russia's invasion of Ukraine looks like on the ground
Photos: What Russia's invasion of Ukraine looks like on the ground Lata Mangeshkar (1929-2022): A pictorial tribute to the 'Nightingale of India'
Lata Mangeshkar (1929-2022): A pictorial tribute to the 'Nightingale of India' PM Modi unveils 216-feet tall Statue of Equality in Hyderabad (PHOTOS)
PM Modi unveils 216-feet tall Statue of Equality in Hyderabad (PHOTOS)The Badminton Association of India (BAI) has announced a 14-member-strong India squad for
- Men’s Sr Hockey Nationals to be played in division-based format from April 4
- Mensik denies Djokovic 100th title in Miami final
- KIPG: Son of a vegetable vendor, Bihar’s Jhandu Kumar eyes Worlds, 2028 Paralympics
- Hardik Singh credits hard work and team unity for receiving HI Midfielder of the Year award
- Djokovic, Alcaraz land in same half of Miami draw
Sputnik V manufacturing to start soon in India: V.K. Paul Last Updated : 27 May 2021 07:14:33 PM IST 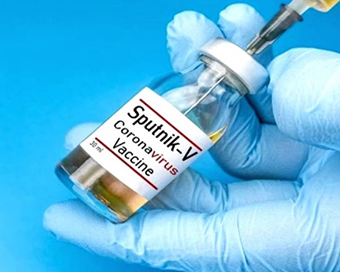
The manufacturing of Russia's Sputnik V Covid-19 vaccine will start soon in India as the country has accomplished technology-transfer to Indian companies, said Dr Vinod K. Paul, chair of the National Expert Group on Vaccine Administration for Covid-19 (NEGVAC) on Thursday.
Paul made the announcement while clearing the myth that the Centre is not doing enough to buy vaccines from abroad.Paul said the Central government has also proactively eased entry of vaccines approved by the US FDA, EMA, UK's MHRA and Japan's PMDA, and WHO's Emergency Use Listing into India in April.He said these vaccines will not need to undergo prior bridging trials as the provision has now been further amended to waive off the trial requirement altogether for the well-established vaccines manufactured in other countries."No application of any foreign manufacturer for approval is pending with the drugs controller," Paul, Member (Health) in NITI Aayog, further said.Paul said that Sputnik vaccine trials got accelerated and with timely approval, Russia has already sent two tranches of vaccines and "accomplished tech-transfer to our companies that would start manufacturing very soon".The declaration comes after the developers of Russia's Sputnik V Covid-19 vaccine and Panacea Biotec on Monday announced that full-scale production of the doses in India will start this summer.The Russian Direct Investment Fund (RDIF), or Russia's sovereign wealth fund, has tied up with Indian pharmaceutical firms such as Gland Pharma, Hetero Biopharma, Panacea Biotec, Stelis Biopharma and Virchow Biotech to make more than 850 million doses a year.Sputnik V has been granted approval for emergency use by India's drug controller. The Russian vaccine was registered in India under the emergency use authorisation procedure on April 12 and use of the Russian vaccine started on May 14.RDIF and Panacea Biotec have agreed to produce 100 million doses a year of Sputnik V.Sputnik V has so far been registered in 66 countries with a total population of more than 3.2 billion. RDIF and Gamaleya Center have said the efficacy of Sputnik V is 97.6 per cent, based on analysis of data on coronavirus infection rate among those vaccinated in Russia with both doses of Sputnik V from December 5 last year to March 31 this year.Paul elaborated that the Central government has continuously remained engaged with all the major international vaccine manufacturers right from mid-2020 and multiple rounds of discussions have happened with Pfizer, Johnson & Johnson and Moderna."We reiterate our request to all international vaccine makers to come and make in India, for India and for the world," Paul said, adding "we need to understand that buying vaccines internationally is not similar to buying 'off the shelf' items"."The government offered all assistance to have them supply and or manufacture their vaccines in India. However, it is not that their vaccines are available in free supply."Paul later clarified those vaccines are in limited supply globally, and companies have their own priorities, game-plans and compulsions in allocating finite stocks."They also give preference to countries of their origin just as our own vaccine makers have done unhesitatingly for us," said Paul, adding as soon as Pfizer indicated vaccine availability, the central government and the company are working together for the earliest possible import of the vaccine.Paul said the Central government has proactively eased entry of vaccines approved by the US FDA, EMA, UK's MHRA and Japan's PMDA, and WHO's Emergency Use Listing into India in April."These vaccines will not need to undergo prior bridging trials. The provision has now been further amended to waive off the trial requirement altogether for the well-established vaccines manufactured in other countries. No application of any foreign manufacturer for approval is pending with the drugs controller."IANS New Delhi For Latest Updates Please-
Join us on
Follow us on








172.31.16.186