Gallery
 PM Modi visit USA
PM Modi visit USA Only the mirror in my washroom and phone gallery see the crazy me : Sara Khan
Only the mirror in my washroom and phone gallery see the crazy me : Sara Khan Karnataka rain fury: Photos of flooded streets, uprooted trees
Karnataka rain fury: Photos of flooded streets, uprooted trees Cannes 2022: Deepika Padukone stuns at the French Riviera in Sabyasachi outfit
Cannes 2022: Deepika Padukone stuns at the French Riviera in Sabyasachi outfit Ranbir Kapoor And Alia Bhatt's Wedding Pics - Sealed With A Kiss
Ranbir Kapoor And Alia Bhatt's Wedding Pics - Sealed With A Kiss Oscars 2022: Every Academy Award Winner
Oscars 2022: Every Academy Award Winner Shane Warne (1969-2022): Australian cricket legend's life in pictures
Shane Warne (1969-2022): Australian cricket legend's life in pictures Photos: What Russia's invasion of Ukraine looks like on the ground
Photos: What Russia's invasion of Ukraine looks like on the ground Lata Mangeshkar (1929-2022): A pictorial tribute to the 'Nightingale of India'
Lata Mangeshkar (1929-2022): A pictorial tribute to the 'Nightingale of India' PM Modi unveils 216-feet tall Statue of Equality in Hyderabad (PHOTOS)
PM Modi unveils 216-feet tall Statue of Equality in Hyderabad (PHOTOS)Hockey India has announced a 54-member core probable squad for the upcoming senior men’s
- Satwik-Chirag return as BAI names 14-strong squad for BWF Sudirman Cup Finals 2025
- Men’s Sr Hockey Nationals to be played in division-based format from April 4
- Mensik denies Djokovic 100th title in Miami final
- KIPG: Son of a vegetable vendor, Bihar’s Jhandu Kumar eyes Worlds, 2028 Paralympics
- Hardik Singh credits hard work and team unity for receiving HI Midfielder of the Year award
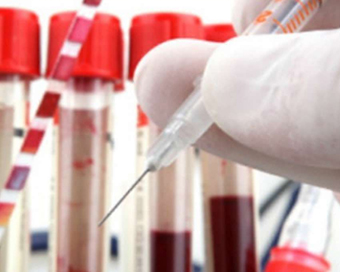
Zydus Cadila vaccines to begin commercial rollout from mid-September Last Updated : 21 Aug 2021 03:34:00 PM IST 
A day after getting the emergency use authorisation (EUA) in India, Zydus Cadila has said that the commercial rollout for the vaccine will start from mid-September.
The pharma major informed about the future rollout plan in a virtual press conference held on Saturday.Zydus' Managing Director Sharvil Patel, said: "Our vaccine is 66 per cent effective against the delta variant, with higher antibody levels in the age group of 12 years and 18 years."So far, 28,000 volunteers have participated in the trial of the vaccine at more than 50 locations. The results of the first and second trials have been published in the Lancet for scientific research and scrutiny, the results of the third phase trial will take 2 to 3 months to come."So far, all the volunteers above the age of 12 years have participated in the trial, while 1,400 children whose age is less than 12 years have applied for the trial. No adverse impacts were observed during the vaccine trials, we have also conducted trials on Delta and Delta Plus variants."Talking about the vaccine price, he said that there will be more clarity about the price of the vaccine by next week.On the question of mass supply of the vaccine, Patel said that the supply of vaccines will begin by mid-September.He further said that the company is aiming at increasing the production of the vaccine up to 1 crore doses per month from the month of October with the help of the new production plant.Patel added that Zydus vaccine is the world's first vaccine to be delivered with a microneedle injector manufactured with DNA technology that can reduce injection costs and adverse effects by 25 per cent.On distribution policy, he said: "Our vaccine can be stored at 25 AoC for 3 months. Our effort is to make 30 to 50 lakh vaccine doses in the coming few days, we are trying to produce 10 million doses per month from October."Zydus Cadila's three-dose indigenous Covid-19 vaccine ZyCoV-D is the world's first DNA-based jab that can be administered to people aged 12 and above.Zydus Cadila received the EUA in India on Friday.IANS New Delhi For Latest Updates Please-
Join us on
Follow us on








172.31.16.186