Gallery
 PM Modi visit USA
PM Modi visit USA Only the mirror in my washroom and phone gallery see the crazy me : Sara Khan
Only the mirror in my washroom and phone gallery see the crazy me : Sara Khan Karnataka rain fury: Photos of flooded streets, uprooted trees
Karnataka rain fury: Photos of flooded streets, uprooted trees Cannes 2022: Deepika Padukone stuns at the French Riviera in Sabyasachi outfit
Cannes 2022: Deepika Padukone stuns at the French Riviera in Sabyasachi outfit Ranbir Kapoor And Alia Bhatt's Wedding Pics - Sealed With A Kiss
Ranbir Kapoor And Alia Bhatt's Wedding Pics - Sealed With A Kiss Oscars 2022: Every Academy Award Winner
Oscars 2022: Every Academy Award Winner Shane Warne (1969-2022): Australian cricket legend's life in pictures
Shane Warne (1969-2022): Australian cricket legend's life in pictures Photos: What Russia's invasion of Ukraine looks like on the ground
Photos: What Russia's invasion of Ukraine looks like on the ground Lata Mangeshkar (1929-2022): A pictorial tribute to the 'Nightingale of India'
Lata Mangeshkar (1929-2022): A pictorial tribute to the 'Nightingale of India' PM Modi unveils 216-feet tall Statue of Equality in Hyderabad (PHOTOS)
PM Modi unveils 216-feet tall Statue of Equality in Hyderabad (PHOTOS)The Badminton Association of India (BAI) has announced a 14-member-strong India squad for
- Men’s Sr Hockey Nationals to be played in division-based format from April 4
- Mensik denies Djokovic 100th title in Miami final
- KIPG: Son of a vegetable vendor, Bihar’s Jhandu Kumar eyes Worlds, 2028 Paralympics
- Hardik Singh credits hard work and team unity for receiving HI Midfielder of the Year award
- Djokovic, Alcaraz land in same half of Miami draw
Pfizer Covid vaccine safe, generates immune responses in kids aged 5-11 Last Updated : 20 Sep 2021 06:38:11 PM IST 
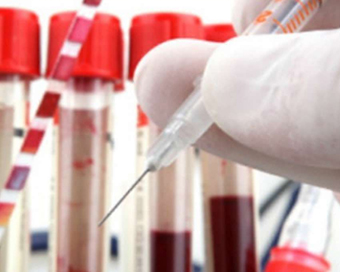
The mRNA Covid vaccine developed by Pfizer has been found to be safe, well-tolerated, and has shown robust neutralising antibody responses in children aged 5 to 11 years, the US drug maker said on Monday.
The results are based on the first-ever trial of any Covid-19 jabs in kids under 12 years of age, Pfizer said in a statement.The Phase 2/3 study enrolled 2,268 children who were 5 to 11 years of age and were given a two-dose regimen of 10 micrograms 21 days apart. A smaller dose than the 30 microgram dose is used for people 12 and older.The antibody responses in the participants given 10 microgram doses were comparable to those recorded in a previous Pfizer-BioNTech study in people 16 to 25 years of age immunised with 30 microgram doses."Over the past nine months, hundreds of millions of people ages 12 and older from around the world have received our Covid-19 vaccine. We are eager to extend the protection afforded by the vaccine to this younger population, subject to regulatory authorisation, especially as we track the spread of the Delta variant and the substantial threat it poses to children," Pfizer Chairman and Chief Executive Officer, Albert Bourla, said in the statement.Further, the Covid-19 vaccine was found to be well tolerated, with side effects generally comparable to those observed in participants 16 to 25 years of age.The data will be submitted "as soon as possible" to the US Food and Drug Administration (FDA), the European Medicines Agency (EMA) and other regulators, Pfizer said.The company is also expected to soon share the result of the trial on children aged 2-5 years of age and children 6 months to 2 years of age.The new data comes as children made up an increased portion of cases in many US states and parents are anxious to get their children vaccinated, especially as schools reopen."Since July, pediatric cases of Covid-19 have risen by about 240 per cent in the US -- underscoring the public health need for vaccination. These trial results provide a strong foundation for seeking authorisation of our vaccine for children 5 to 11 years old, and we plan to submit them to the FDA and other regulators with urgency," Bourla said.So far, of the vaccines available in the US, only the Pfizer-BioNTech shots have been cleared by the FDA for people as young as 12, while Moderna's and Johnson & Johnson's vaccines have been authorised for adults.IANS New York For Latest Updates Please-
Join us on
Follow us on








172.31.16.186