Gallery
 PM Modi visit USA
PM Modi visit USA Only the mirror in my washroom and phone gallery see the crazy me : Sara Khan
Only the mirror in my washroom and phone gallery see the crazy me : Sara Khan Karnataka rain fury: Photos of flooded streets, uprooted trees
Karnataka rain fury: Photos of flooded streets, uprooted trees Cannes 2022: Deepika Padukone stuns at the French Riviera in Sabyasachi outfit
Cannes 2022: Deepika Padukone stuns at the French Riviera in Sabyasachi outfit Ranbir Kapoor And Alia Bhatt's Wedding Pics - Sealed With A Kiss
Ranbir Kapoor And Alia Bhatt's Wedding Pics - Sealed With A Kiss Oscars 2022: Every Academy Award Winner
Oscars 2022: Every Academy Award Winner Shane Warne (1969-2022): Australian cricket legend's life in pictures
Shane Warne (1969-2022): Australian cricket legend's life in pictures Photos: What Russia's invasion of Ukraine looks like on the ground
Photos: What Russia's invasion of Ukraine looks like on the ground Lata Mangeshkar (1929-2022): A pictorial tribute to the 'Nightingale of India'
Lata Mangeshkar (1929-2022): A pictorial tribute to the 'Nightingale of India' PM Modi unveils 216-feet tall Statue of Equality in Hyderabad (PHOTOS)
PM Modi unveils 216-feet tall Statue of Equality in Hyderabad (PHOTOS)The Badminton Association of India (BAI) has announced a 14-member-strong India squad for
- Men’s Sr Hockey Nationals to be played in division-based format from April 4
- Mensik denies Djokovic 100th title in Miami final
- KIPG: Son of a vegetable vendor, Bihar’s Jhandu Kumar eyes Worlds, 2028 Paralympics
- Hardik Singh credits hard work and team unity for receiving HI Midfielder of the Year award
- Djokovic, Alcaraz land in same half of Miami draw
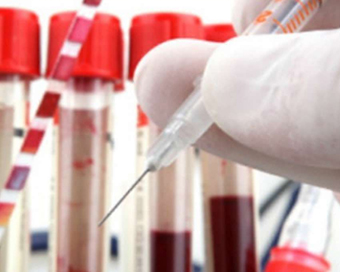
Pfizer says its Covid vaccine 90.7% effective in kids aged 5-11 Last Updated : 23 Oct 2021 10:32:48 AM IST 
Pfizer and its partner BioNTech said their Covid-19 vaccine is safe and 90.7 per cent effective against symptomatic coronavirus in children aged 5 to 11.
The companies revealed the data in a document posted on Friday ahead of a meeting of advisers to the Food and Drug Administration (FDA) scheduled for October 26.Pfizer and BioNTech are applying FDA emergency use authorization (EUA) of a two-dose regimen of their 10-microgram dose for children of the mentioned age group.The two doses would be administered three weeks apart.The data show that the two-dose primary series of the vaccine given to children from 5 to less than 12 years of age confers a high degree of protective efficacy against Covid-19 during a period when the Delta variant of concern predominates in the US.The FDA's Vaccines and Related Biological Products Advisory Committee is scheduled to meet on October 26 to discuss whether to authorise the jab.If authorised, it would be the first Covid-19 vaccine for younger children.The Pfizer-BioNTech vaccine is currently fully approved for people ages 16 and older, and has an EUA for children aged 12 to 15.Friday's announcement comes just days after the White House unveiled a plan to roll out Covid-19 vaccines for children aged from five to 11."In anticipation of the FDA's independent advisory committee meeting on October 26 and the CDC's (Centers for Disease Control and Prevention) independent advisory committee meeting on November 2-3, today the Biden Administration is announcing a plan to ensure that, if a vaccine is authorized for children ages 5-11, it is quickly distributed and made conveniently and equitably available to families across the country," the White House said in a statement on Wednesday.The latest plan "will mobilise a comprehensive effort across the public and private sectors to ensure that we have the supply, the sites, and the support needed to get our nation's children vaccinated and protected against the virus", it added.IANS Washington For Latest Updates Please-
Join us on
Follow us on








172.31.16.186